Calculations in Organic Chemistry
Hi friends,
If you are studying Organic Chemistry or doing a job related
to Organic Chemistry, then you may encounter with the need for different kinds
of calculations. These calculations are absolutely essential before charging any
kind of Organic reactions in laboratory. If you want to learn these
calculations very easily, then this blog post is for you………….
Mole percentage -
Multiplying
the mole fraction by 100 gives mole percent. When a chemical reaction is
conducted, the limiting reagent is often set to be considered 100 mol % [i.e.
mole percent] When another reagent is required the amount can be expressed as
either equivalents [For example, 1.2 equivalents] or as mol % [120 mol % is
same as 1.2 equivalents]
Limiting reagent -
The limiting reagent, also known as
the limiting reactant, is the substance which is totally consumed when the
chemical reaction is completed. The amount of product formed is limited by this
reagent since the reaction cannot proceed further without it. The other
reagents may be present in excess of quantities required to react with the
limiting reagent. The limiting reagent must be identified in order to calculate
the percentage yield of a reaction. Since the theoretical yield is defined as
the amount of product obtained when the limiting reagent reacts
completely. A limiting reagent is a
chemical reactant that limits the amount of product that is formed. The
limiting reagent gives the smallest yield of product calculated from the
reagents (reactants) available. This smallest yield of product is called the
theoretical yield. To find the limiting reagent and theoretical yield, carry
out the following procedure:
- Find the moles of each reactant present.
- Calculate the moles of a product formed from each mole of reactant.
- Identify the reactant giving the smaller number of moles of product. This reactant is the Limiting Reagent.
- Calculate the grams of product produced by the Limiting Reagent. This is the theoretical yield.
Theoretical yield -
Since,
Therefore,
Percentage yield -
Density -
Volume of solvent - Many times we come across the situation where we needs to find out the amount of solvent needed before charging the Organic Reactions. The below mentioned formula will help you to find out the required amount of solvent for the charging the general Organic reactions.
Equimolar = of or relating to an equal number of moles.



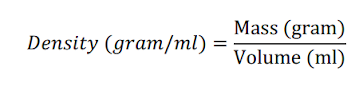

very informative......
ReplyDelete